Intelon Optics introduces BOSS®, the first commercially available CE-marked and FDA-cleared Brillouin non-contact ophthalmic scanner to measure ocular-tissue biomechanics in real-time high-resolution 3D.
Enables fast and easy non-contact scans of the cornea and crystalline lens via automated optical scanning.
Adds an extra dimension of biomechanics to complement traditional tools for a more comprehensive assessment and helps improve treatment decisions and early diagnoses of eye diseases.
Supported by over 40 peer-reviewed publications exploring the use of Intelon's technology in ophthalmic research and clinical studies.
CLINICAL APPLICATIONS
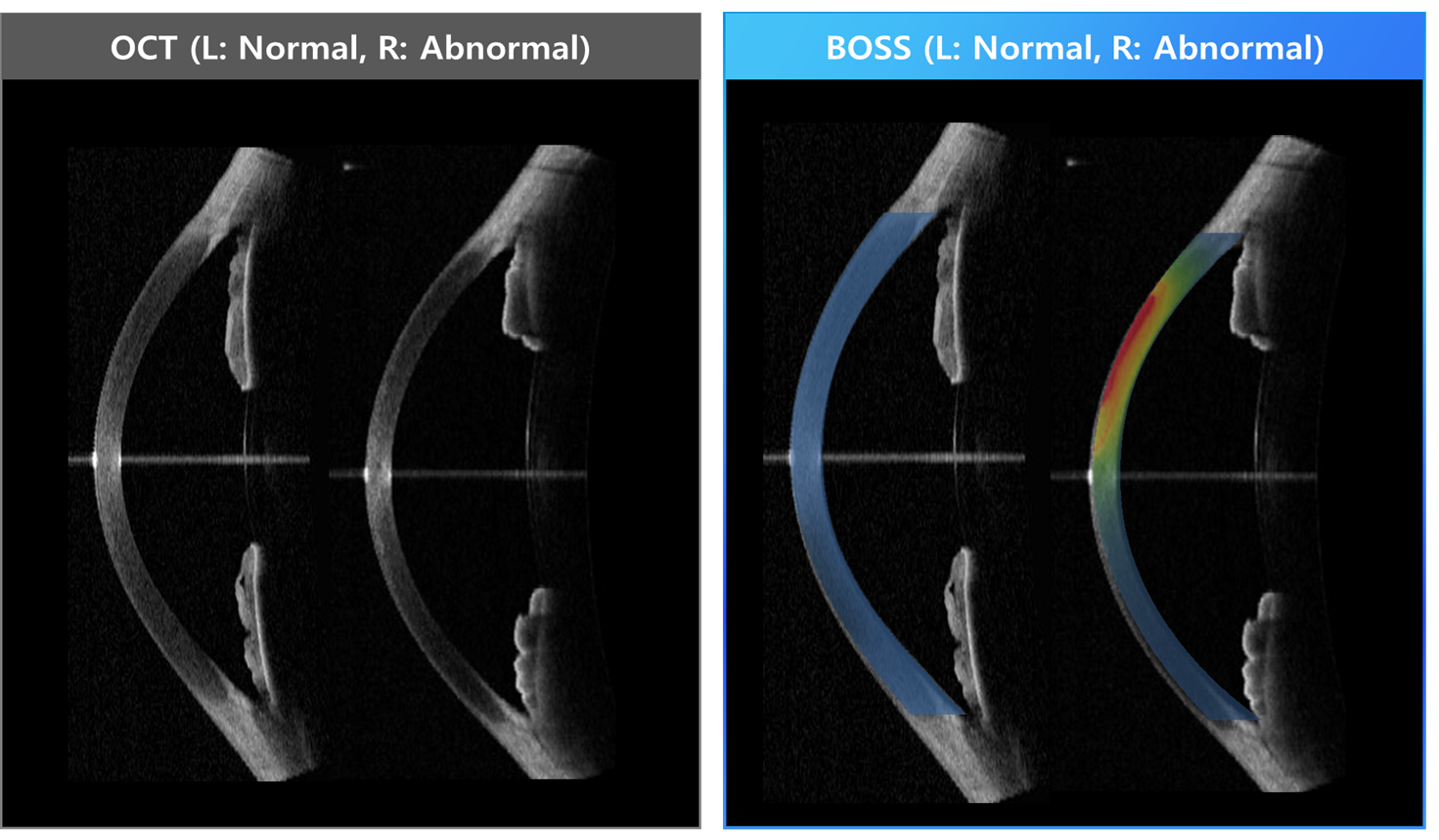
BOSS® provides insight into subtle biomechanical changes in ocular tissues not evident with OCT alone.
Such data has been explored in published research for potential applications, including:
Investigating early biomechanical changes associated with corneal disease,
Supporting screening/optimization for refractive surgery planning,
Evaluating corneal crosslinking protocols in clinical studies, and
Assessing post-operative biomechanical response after cataract surgery.
DATA ANALYTICS
BOSS® AI offers advanced analysis of biomechanical data, supporting clinicians with additional insights during treatment planning. In an internal feasibility study, use of BOSS AI data in refractive surgery planning showed a potential improvement in treatment planning accuracy. These findings are preliminary and not intended to represent established clinical outcomes.
ACADEMIC ARTICLES RELATED TO BOSS®
Intelon Optics developed BOSS® over the last decade with top universities and clinics; there are now 45 peer-reviewed articles in ophthalmology covering Intelon’s Brillouin technology. Research indicates that biomechanics adds an extra dimension to improve clinical outcomes. Click the button below to see a complete list of peer-reviewed ophthalmology articles related to BOSS® technology.
Where to buy
What the experts say