Upcoming events.

Intelon @ ASCRS 2026
Join Intelon Optics at booth #1464 at ASCRS in Washington D.C. from April 10-13 where our team will be happy to introduce you to the BOSS Brillouin Optical Scanning System.

Intelon @ World Keratoconus Congress 2026
Join Intelon Optics at the third World Keratoconus Congress in Florence, Italy from April 16-18 where our team will be happy to introduce you to the BOSS Brillouin Optical Scanning System.

Intelon @ ARVO 2026
Meet with Intelon Optics’ experts at ARVO 2026 to learn more about ocular biomechanics and the BOSS Brillouin Optical Scanning System.

Intelon @ ESCRS 2026
Join Intelon Optics at ESCRS in London, England from September 11-15 where our team will be happy to introduce you to the BOSS Brillouin Optical Scanning System.

Intelon @ AAO 2026
Join Intelon Optics at AAO in New Orleans, LA from October 9-12 where our team will be happy to introduce you to the BOSS Brillouin Optical Scanning System.

Intelon @ KCXL International Experts’ Meeting
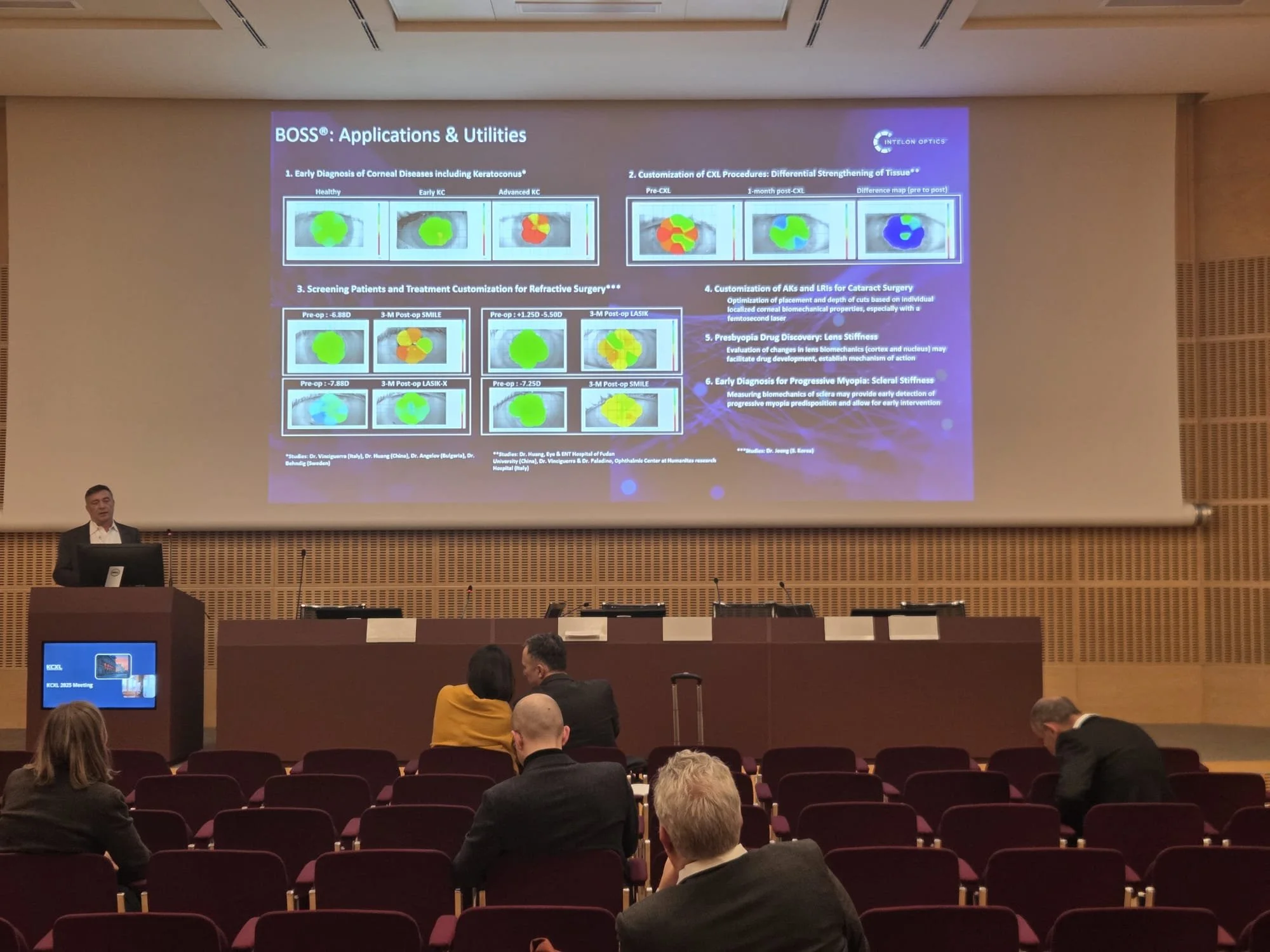
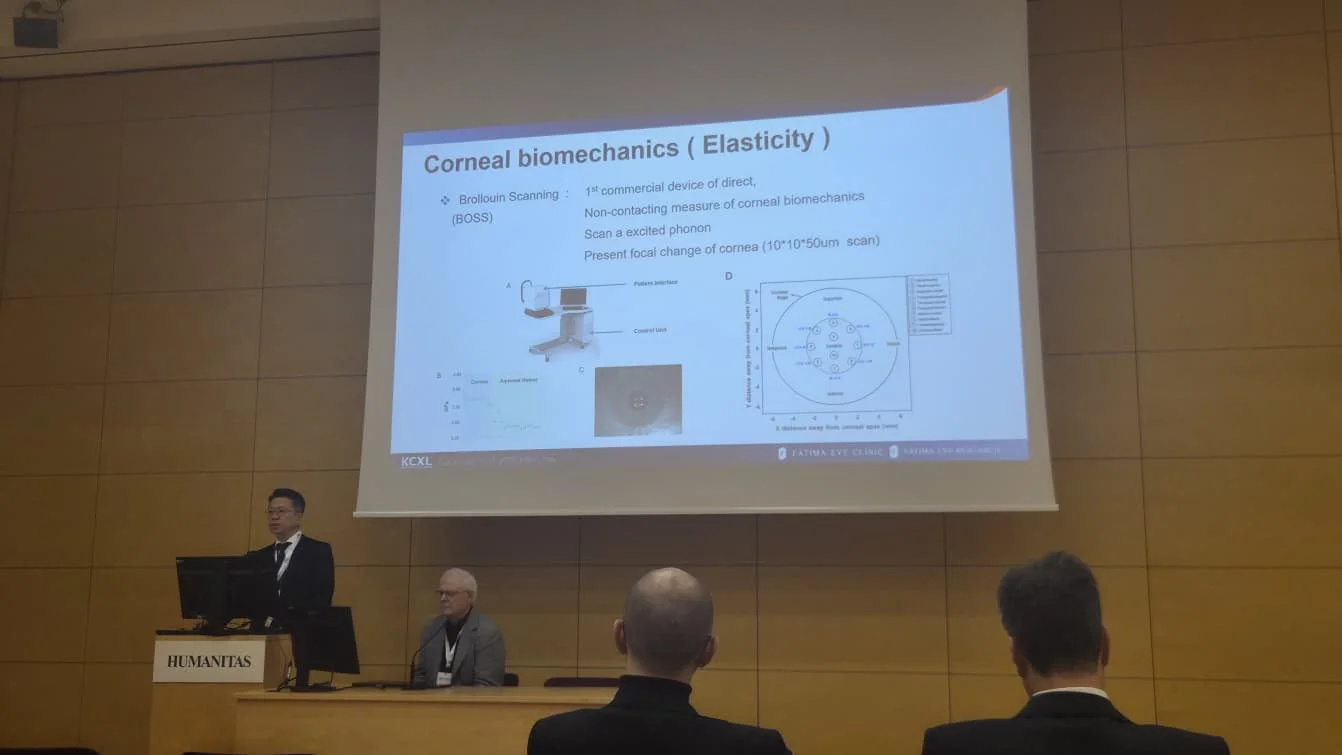
KCXL Experts Meeting is a leading international forum dedicated to advancing knowledge and clinical practice in keratoconus and corneal cross-linking (CXL). The Intelon Optics team had a successful event in Milan, Italy, further advancing the understanding of the BOSS Brillouin Optical Scanning System and corneal biomechanics amongst the ophthalmic community.

Intelon @ AAO 2025
Join us at AAO 2025!
We’ll be at the American Academy of Ophthalmology meeting in Orlando, FL, October 17–20, 2025.
Stop by booth #1034 — we’d love to see you!

Intelon @ ESCRS 2025
European Society of Cataract & Refractive Surgery held in Copenhagen, Denmark from September 12-16, 2025

Intelon @ ARVO 2025
Association for Research in Vision and Ophthalmology will be held in Salt Lake City, UT (USA) from May 4-8, 2025 .

Intelon @ ASCRS 2025
American Society of Cataract & Refractive Surgery will be held in Los Angeles, CA (USA) from April 25-28, 2025.

Intelon has received clearance from the US FDA for BOSS!
Intelon Optics is pleased to announce that it has received clearance from the U.S. Food and Drug Administration (FDA) for its innovative anterior eye segment analysis device. This groundbreaking device is the first to leverage the principles of Brillouin technology to assess the biomechanics of the eye.
The Intelon BOSS device is intended for imaging the cornea and crystalline lens, as well as measuring the elastic properties of these ocular structures. "We are excited to have received FDA clearance for our BOSS device," said Dimitri, COO of Intelon Optics. "BOSS offers eyecare professionals critical insights into the elastic properties of the cornea and crystalline lens tissues, displayed as a map and/or individual axial scans"
Extensive testing has confirmed that the BOSS device is both safe and effective for measuring the longitudinal elastic modulus of the cornea and crystalline lens. The device consistently delivers repeatable and reproducible results, making it a valuable tool for eye care professionals.
This FDA clearance marks a significant milestone for Intelon Optics, enabling the BOSS system to expand its market presence with its cutting-edge technology. The company remains committed to ongoing research and development to further enhance the BOSS device's capabilities. Intelon Optics plans to commercialize the BOSS system later this year.









INTELON RECOGNIZED AS A 2018 GREAT AWARD WINNER
Read the Rochester Business Journal and WHAM13 News articles